Immunoassay techniques are methods for the sensitive and specific quantitative detection of chemical substances such as hormones, drugs and specific proteins that utilize the highly specific binding between an antigen or hapten and homologous antibodies.
Traditional immunoassays such as the enzyme-linked immunosorbent assay (ELISA) are able to measure the presence or absence of only one analyte per reaction. Multiplex immunoassays measure dozens of different analytes in a single reaction. This is particularly beneficial for precious samples, and when only a small volume is collected for analysis. However, due to an increasing demand for more advanced immunoassay techniques capable of extracting large amounts of several analytes from a limited sample volume multiplex immunoassay was developed and introduced. Multiplex immunoassays are low-cost, short-time, flexible, and high throughput methods for simultaneous detection of multiple analytes from one single sample without affecting the sensitivity or specificity of final yield.
The multiplex immunoassays employ traditional immunoassay methods (such as ELISA) which rely on the specificity of antigen-antibody binding (i.e. either antibodies or proteins/peptides are used as binder molecules to capture circulating proteins or antibodies, respectively). Nevertheless, the traditional ELISA relies on an enzymatic reaction for the detection system while most modern multiplex assays rely on fluorescent probes.
There are two general categories of multiplex immunoassays, and they are categorized based on the type of surface on which the antibodies or antigens are bound:
- Planar microarray (protein chips) on which the targets for each analyte are spatially separated
- Suspension array (microparticle or bead microarrays), which are antigen-/antibody-bound beads containing an identifying dye
The multiplex immunoassay magnetic beads, among novel multiplex immunoassay technologies under development, have been under investigation. Magnetic beads were introduced in biological detection and separation systems in the 1970s. Biomolecule-conjugated beads when coupled to affinity molecules (such as specific antibodies) can function as sensitive biological biosensors with a very high separation capability compared to the other techniques. Magnetic beads have biocompatibility, stability and an easily modifiable surface with biomolecules, and can be manipulated on a microsystem. Since the magnetic beads have a large surface area per unit volume, it can provide relatively large binding sites for biochemical reactions. After binding their target analytes, by applying a magnetic field to the containing vessel the magnetic beads can be easily separated from the rest of the sample.
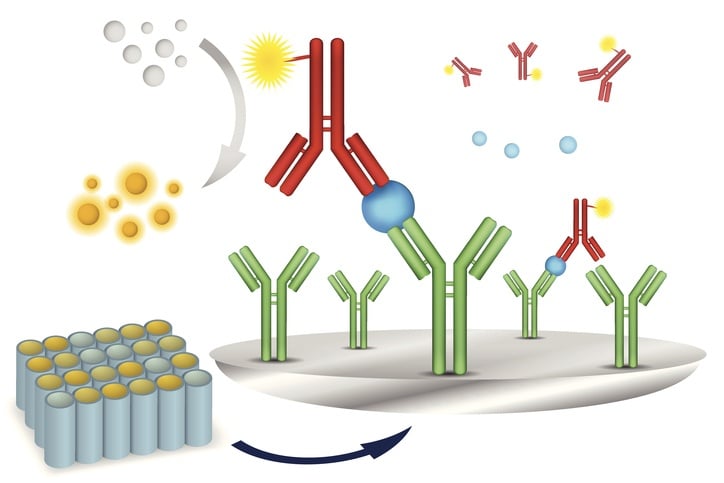
The multiplex immunoassay beads
The bead based multiplex immunoassay requires beads, typically made of polystyrene, that are impregnated with a mixture of red and near-infrared dyes. Manufacturers can create 100 or more uniquely identifiable bead sets by altering the ratio of these dyes. Each analyte to be measured is assigned to a particular bead set, and antibodies are immobilized on the surface. The beads are incubated with the sample for an appropriate time to enable binding between the antibodies and antigens. A secondary probe is also introduced during this incubation period.
Principles of multiplex immunoassays
In suspension assays, the capture ligands are immobilized onto color or size-coded microspheres and specific fluorescent signals are detected using flow cytometry. The suspension assay employs plastic microbeads infused with a chemiluminescent/fluorescent dye and an activated surface linking it to a specific capture antibody. The detection antibodies with chemiluminescent/fluorescent reporter are added upon completion of incubation and washing stages. Several beads are prepared, each with a separate capture antibody according to the analyte and a unique fluorescent signature that enables identification. Each bead accommodates a “sandwich” consisting of a captured target analyte and similar reporter-conjugated detection antibody. The bead analyte reporter constructs are analyzed in a flow chamber where lasers excite the reporters and the emitted light is collected by a series of detectors for quantitative analysis.
The secondary identification probe for multiplex immunoassays
The secondary probe consists of a fluorescent probe conjugated to an antigen or antibody complementary to the analyte. This antigen or antibody is the same type that is conjugated to a bead. As an example, say we are doing an assay for the presence of specific antibodies in a clinical sample. One bead set will be bound with antibodies specific to antigen A. Another bead set will be bound with antibodies specific to antigen B. A third bead set will be bound with antibodies specific to antigen C, and so on. Additionally, free antigens specific to type A, B, and C are bound to green fluorescent probes. All of these components are mixed with the sample. If antigen A is in the sample it will bind to the bead set bound with antibody A, and the free-floating probe-conjugated antibody will also bind to the same antigen and form a sandwich. This joins the red fluorescent bead and the secondary green fluorescent probe together into one conjugated system. If antigen B is present in the sample it will form its own conjugated system. If antigen C is not present in the sample, then the red fluorescent bead will not be conjugated to a green fluorescent probe because there is no antigen to link the two together.
Quantification by flow cytometry
A flow cytometer is used for data acquisition. The flow cytometer sends a stream of beads through laser beams one bead at a time. One laser is red and the other is green. The red laser identifies the specific analyte being measured by identifying the unique color of the bead set. The green laser identifies whether or not the secondary probe was conjugated to the bead via the analyte of interest. In the example above, beads conjugated to antigens A and B will fluoresce green, while beads from set C will not.
Superparamagnetic beads for multiplex immunoassays
Superparamagnetic beads are being introduced to multiplex immunoassays. The magnetic beads are coated with a dye-impregnated polymer in order to have both magnetic and fluorescent properties. The magnetic property allows for automated washing steps to be added to the protocol to remove contaminants or unbound probes. This can help the flow cytometry process proceed smoothly. Alternatively, the magnetic beads allow other quantification methods that don't require an expensive flow cytometer and lasers. The magnetic beads can be drawn to a surface and immobilized while an LED-based quantification system measures the fluorescent signals.
Other applications for multiplex assays
The fluorescent bead-based multiplex system is also useful for the detection of nucleic acids. In this case, a single strand sequence is covalently attached to the bead surface. This sequence is complementary to the target sequence. The bead is included in a polymerase chain reaction of the sample, and will be extended by DNA polymerase. Some of the bases used by the DNA polymerase are modified to contain a binding site for a fluorescent probe. The presence of the target sequence is therefore indirectly measured by the multiplex system.
The developers of this bead-based-fluorescent multiplex assay also sell premade kits containing beads conjugated with an array of common targets. If a premade kit doesn’t contain all of the markers to meet your unique experimental goals, it is easy to order a custom-made kit. There is no need for the researcher to conjugate each bead set his or herself. This service makes it quick and easy to perform multiplex assays on a variety of targets.
Related news