Biomagnetic separation used to take place in academic labs, but recently it has become a very industrial application. As processes are scaled up and volumes increase, the investment required for each batch is larger, but the expected economic return is also larger.
Recent Posts

It is understandably important to end users that every kit within a particular lot have the same properties. In other words, when one is producing lots of material to be used in an IVD kit, one necessarily strives for maximum reproducibility and minimal variability. With standard magnetic separators, it is very difficult to achieve this goal in a magnetic separation process.


Because biomagnetic separation techniques are relatively simple, life science laboratories and industries are quite enamored with them. Indeed, using only magnetic beads and magnetic fields, biomolecules can be captured and extracted from complex media in magnetic bead separation. However, if this application is to be considered practical, it should also be faster than other separation technologies such as chromatography, electrophoresis or centrifugation.

By Lluis M. Martinez, CSO Sepmag
Often when a lab produces a product that becomes popular, the impetus is to move forward and scale up production of that product. The problem is that moving from the production of small lots to full scale production usually produces surprising results. Scaling up is not trivial, and the magnetic separation process is no exception. When one scales up production, results become very inconsistent.


Because biomaterial is expensive, fragile, complex and sometimes rare, biotech companies spend a great deal of time and resources to develop and refine biomaterial production processes. Quality control and standard operating procedure demand that production managers make sure that all technicians and operators know and follow the exact procedures from batch to batch.

What are the problems of the classic magnetic separation process? Typically the classic ways to produce magnetic bead reagents and kits are slow, very high maintenance and costly to run. The three classic techniques, centrifugation, filtration and tangential filtration, are not straightforward techniques.


Consistent lot-to-lot results are achieved with biomagnetic technology only when magnetic bead separation is performed in defined and homogeneous conditions. When homogeneity is realized, separation is reproducible and scalable.

Of course, as in most industries, product consistency is key to the success of the Life Sciences industry. With magnetic bead separation, not only should working conditions be constant over time, but conditions should also be consistent from lot to lot, regardless of the time between production runs. One thing that should always be considered is the quality of the magnet used in the biomagnetic separation devices.

Scientists in academic research labs and pharmaceutical labs perform magnetic separation process with magnetic bead kits for immunoassays and separation science. Doctors, lab technicians and scientists use magnetic beads in IVD kits as molecular diagnostics devices.
Those who use Life Sciences products rightly demand that these products show consistency from batch to batch. In other words, when comparing batches, one should find very little, if any, variability.
Last March 4th, Mr. Jordi Andreu Segura defended his paper “Statistical Mechanics of Superparamagnetic Colloidal Dispersions Under Magnetic Fields”, which was submitted to obtain a PhD degree in Materials Science at the Universidad Autónoma de Barcelona.
One of the major problems of traditional magnetic bead separation technology is aggregation of the magnetic beads. Aggregation decreases the yield and also contributes to variability between batches.

Reproducibility is very important when considering production of in vitro diagnostic kits. As such, there should be good quality control in place that strictly defines the parameters of the assay’s raw materials. For example, magnetic beads, antibodies and buffers should not vary from batch to batch. In addition, these raw materials should all act the same during biomagnetic separation.
Often in life science research and clinical applications, magnetic carriers are utilized to separate or isolate biomolecules from suspension. A biomolecule is coated onto a magnetic bead or is ‘captured’ by a bead and then pulled to a given position in the solution via magnetic forces. In order to establish a robust and reproducible standard operating procedure (SOP), one needs to understand how the magnetic forces are generated and what key parameters need to be controlled.

When a biotech company or other related industry decides to use biomagnetic separation technology, a great deal of time, energy and resources are typically spent by R&D and manufacturing departments to determine the optimal bead to use for their specific applications. Often, however, they overlook one of the major determinants of separation: homogeneity of separation conditions.

Magnetic beads are used by many biotech companies, typically for in vitro diagnostic applications and magnetic bead separation. Because these beads are so widely used, it is very important that every aliquot of every batch has exactly the same properties.
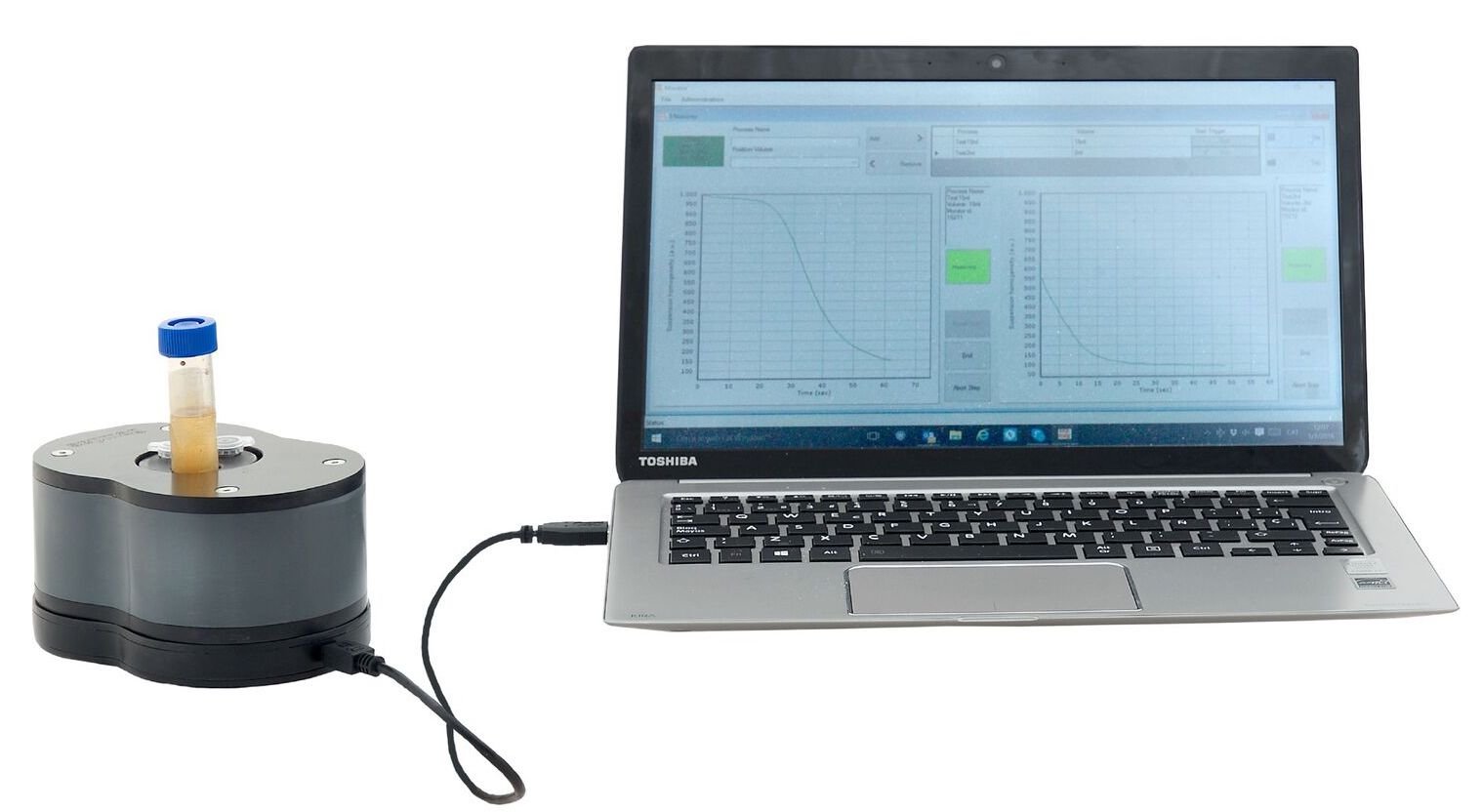
Traditional biomagnetic separation techniques have several drawbacks that affect both the quality and the quantity of the final product.

Biomagnetic separation needs validation in order to ensure reproducibility. The skills necessary to identify the key parameters affecting separation performance, measure those parameters, and enact the appropriate controls are specific and require an excellent background in physics.

Magnetic bead separation is being used in more and more applications (e.g. immunoassays, collection of genetic material, protein purification). Most of these applications are industrialized and therefore require quality control protocols, validation audits and standard operating procedures. Because of the economic implications, production mistakes are not acceptable, so products must demonstrate reproducibility in order to be viable.

The cornerstone of any good production process is the ability to have robustness and reproducibility, especially in the biotech industry and in the magnetic bead separation industry.

Since reproducibility over time is a highly desired trait when using biomagnetic separation, especially when used in the life sciences, it is important to consider all possible disruptions of consistency. Biomagnetic separation devices use permanent magnets which maintain their properties over long periods of time.

Biotech companies such as InVitro Diagnostic aim for 100% reproducibility in every single batch they produce and in every kit test in each batch. In fact, customers expect that they will receive a product that will perform exactly the same as the last time they purchased it. It does not matter what batch the product is or when it was produced.

It is vitally important for life sciences products to be consistent from lot to lot, so two batches of the same product produced in the same way should have little variability. In order to achieve a high level of quality control, one must define a strict standard operating procedure (SOP). In the case of a permanent magnet magnetic bead separation device, conditions are usually very stable and so the main parameter to control is the time the vessel is exposed to the magnetic field during production.

There are no easy ways to bypass steps or simplify the production process of magnetic bead separation. Steps in the production process that seem easy or easily bypassed, turn the production into a nightmare if one attempts to take short cuts. One of these seemingly easy steps is the resuspension step.
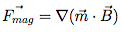
During the development of a magnetic bead separation process, scientists put great effort into reproducing the size of the beads, the magnetic charge on the beads, buffer composition, pH and temperature. What is often overlooked, however, is the importance of homogeneous biomagnetic separation conditions.
This post is about magnetic bead separation and how to validate this process. If you are interested in this topic, and are willing to learn more about it, download our Free Guide The Starting Guide to Validate Biomagnetic Separation Processes:

Separation techniques using magnetic carriers (either beads or particles) are often used in the life sciences to ‘capture’ specific biomolecules. These techniques utilize immunocapture, DNA fragments, or electrical charge in order to specifically target the biomolecule of choice. After magnetic capture of the biomolecule, magnetic forces can separate it from the rest of the milieu. Because of the seeming ease of separation, biomagnetic techniques are used by some as the ‘gold standard’ of separation technology.
The 10th Clinical Laboratory Provider and Blood Transfusion Equipment Expo (CACLP), will be held in the Xi’an Greenland Pico International Convention & Exhibition Center next march. This Trade Show will attract to the city of the Terra Cotta Warriors main Chinese companies as well as major foreign players with interests in the In Vitro Diagnostic sector.

Protein purification using magnetic particles is well established in life science R&D but has not reached commercial acceptance for larger scale applications in bioprocessing. Preparative scale purification of antibodies using magnetic beads is far more time efficient, however existing column chromatography methods are significantly cheaper. To be competitive, the main challenges that need to be addressed for magnetic separations are:

Biomagnetic separation technology is being widely adopted in many biotech and other life science industries. The managers of these industries spend a lot of energy, time and resources choosing the correct beads for their applications, however they often overlook very important variables that need to be controlled pertaining to the magnetic bead separation process.

In Vitro Diagnostic (IVD) Immunoassays are some of the most successful life science magnetic bead and particle research applications that have come to market. The demand for such technology in hospitals and laboratories in particular, has grown dramatically.

It is common knowledge that industries and biotech companies must validate any production process in order to maintain the consistency of their products. In processes that cannot be validated at a high level, lot numbers become very important so that the end user can compare and understand lot to lot variation.
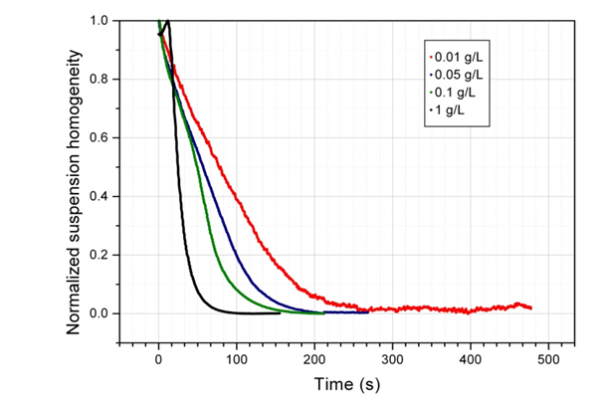
Non-homogeneous biomagnetic separation processes are difficult to validate and standardize. The fact that magnetic beads move at different speeds due to the varied magnetic forces in a non-homogeneous environment makes monitoring the production almost impossible.

A Tool for Synthesis Monitoring and Biomagnetic Applications

SEPMAG will be attending MEDICA on November 14, 15 and 16 in Düsseldorf, Germany. If you are attending too, we will be delighted to meet you and discuss how our proprietary technology may help your company to implement a state of the art biomagnetic separation process.

Sepmag installs a 2L system of its unique biomagnetic separation technology at Resyn Biosciences head laboratory in Pretoria, South Africa, allowing this innovative magnetic bead manufacturer to enhance the reproducibility of production batches, reduce material waste, facilitate the validation process and increase the safety of operation. You can follow it on ResynBio’s twitter and facebook.

EMD Millipore, the manufacturer of ESTAPOR magnetic beads, organizes the Technical Seminar "The Use of MicroSpheres and NanoSpheres in Diagnostics and Life Sciences: Technologies & Applications" in Paris, next September 27th and 28th. The two days have been divided in 3 sessions to allocate 17 technical talks.
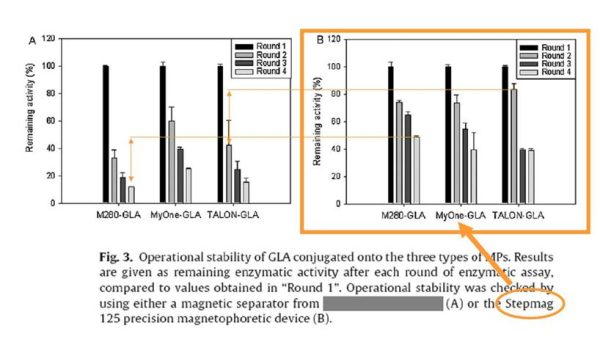
Researchers of the CIBER-BBN, the Institut de Biotecnologia i de Biomedicina and the Department of Genetics and Microbiology of the Universitat Autònoma de Barcelona and Sepmag have recently published the paper “Enzymatic characterization of highly stable human alpha-galactosidase A displayed on magnetic particles” on the Biochemical Engineering Journal.
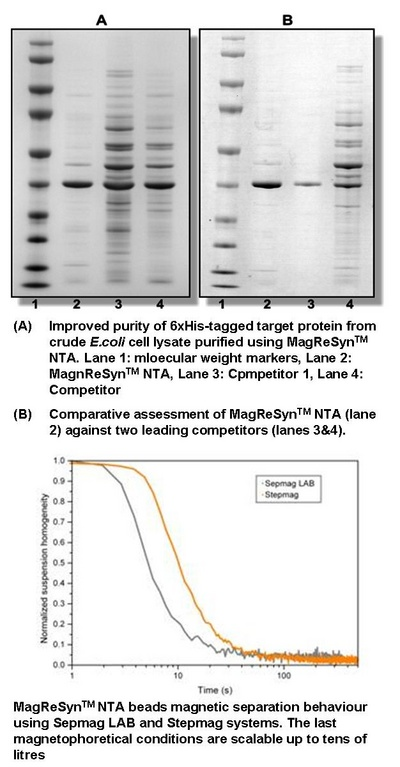
Traditional magnetic beads are comprised of solid microspheres. Unfortunately, binding capacity is limited by the amount of area on the surface of these magnetic beads.

As in every edition since 2008, SEPMAG will be one of the sponsors of the 9th International Conference on the Scientific and Clinical Applications of Magnetic Carriers (http://www.magneticmicrosphere.com) that will be held in Minneapolis, MN, USA from May 22-26, 2012.
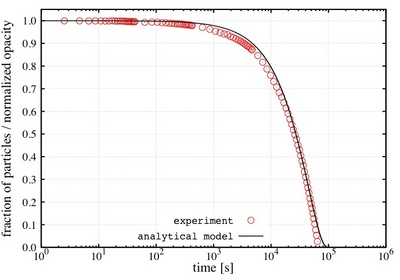
Latest theoretical investigations conducted by researchers from the Institut de Ciencia de Materials de Barcelona and the Grup de Fisica Estadistica de la Universitat Autonoma de Barcelona have shed some light on the key aspects of the magnetophoretic separation process.
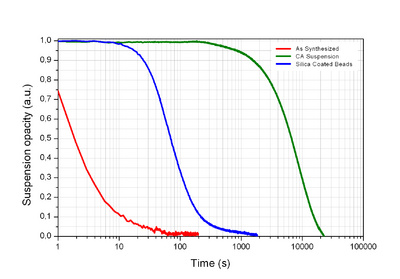
Researchers at Centro de Fisica of the Universidade do Minho (Braga, Portugal) have used Sepmag LAB precision magnetophoresis equipment to characterize the behaviour of magnetic nanoparticle suspensions throughout their synthesis process.