The first four mistakes we described in the last weeks are related to the production process of CLIA IVD-kits. However, even if you get a perfect reproducible, high performant process, it is a last mistake you should avoid. We have frequently see IVD-manufacturers to adopt solutions implying high safety risk for the operators and the equipment.

You should be aware that magnets can generate a high risk of accident, attracting ferromagnetic objects. These objects can be other magnets, scissors, screwdrivers or any magnetizable object. The risk of having parts of the body are trapped between the two objects increases very quickly with the size of the magnets used. For a fridge magnet the worst scenario is that you pinch your finger. For large Rare Earth magnets, careless users can suffer severe injuries, including multiple bone fractures.
For people with pacemakers, the risk is still higher: magnetic fields can interfere with the device, causing malfunctions. That is why you find big danger signs near the MRI areas in hospitals.
But not only people can be harm. Laboratory equipment is also be affected by magnetic fields, especially the ones including magnetic recording media. Many unexperienced people has seen how they Credit Cards and/or they company badges (if magnetic) have been erased. Computers and hard disk drivers can also lost their information, and electronics of many lab systems can also be affected.
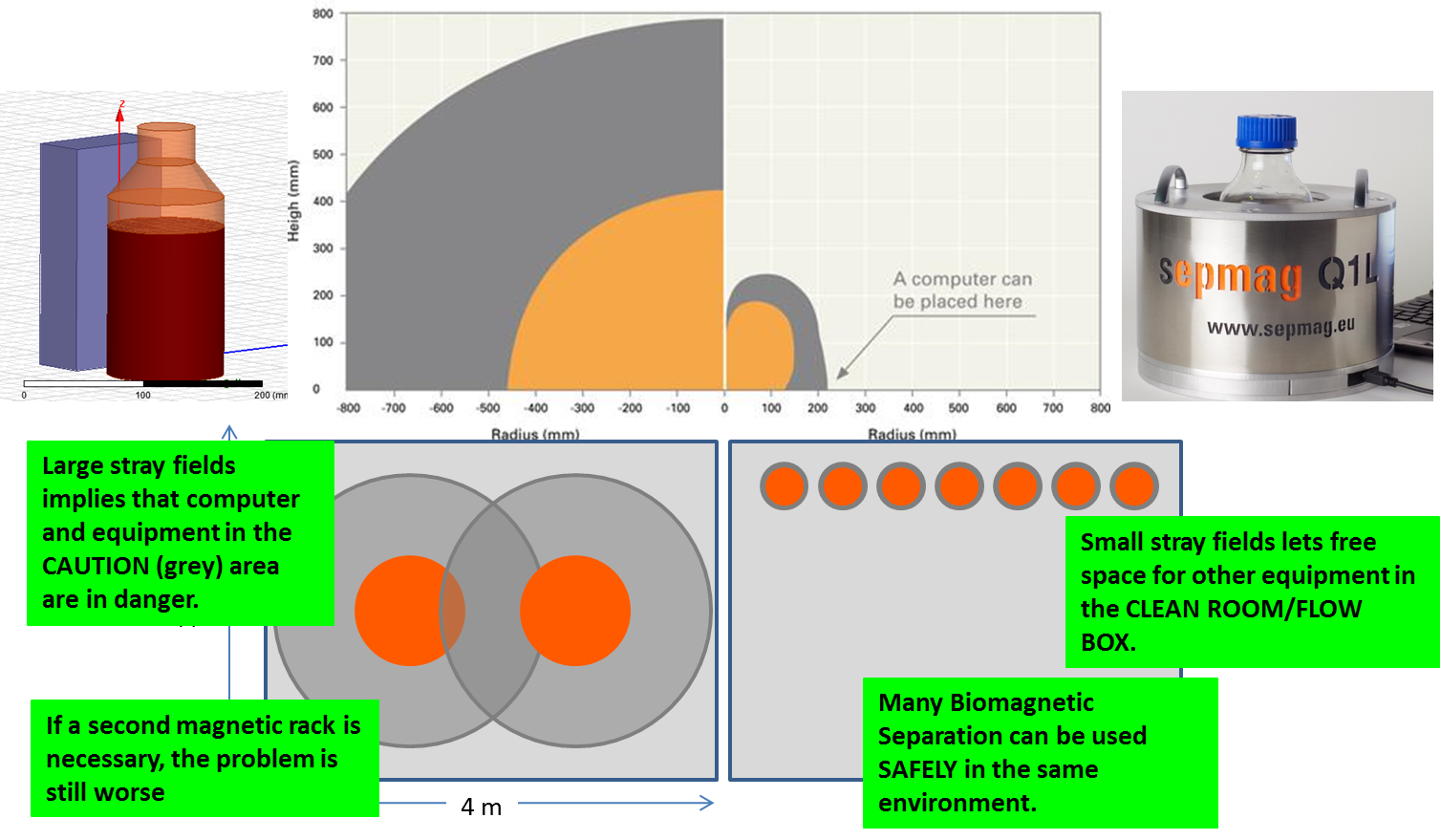
Repercussion of the CAUTION (grey) and DANGER (orange) areas on the Clean Room occupation.
Danger area vs caution area
The need of keeping safety distance has a big repercussion on Clean Room occupation. You need to keep clear a DANGER AREA (Field > 3 mT, 30 Gauss) were it is a risk of accidents by the mechanical attraction between magnets and magnetizable objects. You need also clear a larger CAUTION AREA (Field >0.5 mT or 5 Gauss) where it is a risk of erasing magnetic recording media and pacemakers malfunction.
These safety measures have a large repercussion in Clean Room occupation. As the figures shows, if the magnetic separation rack has been designed without specific attention to safety, the large stray fields will imply that computer and equipment placed inside the CAUTION area are in danger, then a big space should be cleared and no additional equipment can be placed there. If you need to put several magnetic separation devices in the same room, the problem would still be worse.
Remember to download our free guide Five critical mistakes in CLIA IVD-kits manufacturing to learn about all these mistakes:
By contrast, if the Biomagnetic Separation System has been designed keeping the stray fields small, it would be plenty of free space for placing safely other equipment in the CLEAN ROOM/FLOW BOX. Several Advanced Biomagnetic Separation Systems can be placed in an area smaller than the needed for a single classical magnetic separation rack.
Then, how to avoid Mistake #5 (Inappropriate safety precautions when working with magnetic fields)?
First of all, you should ALWAYS comply with the safety measures: respect the recommendations for the Caution and Danger areas.
Then, in addition of the performance as magnetic separator, you should also pay attention to the Stray Magnetic Fields generated by the Magnetic Separation racks. Whenever possible, you should choose Biomagnetic Separation Systems with small Stray fields.
By doing this, you would reduce the risk of accidents, by minimizing the danger area, but also you will saves space on the laboratory/clean room/flow box.
How SEPMAG® may help?
Since 2004, we are helping our customers to avoid these 5 mistakes. Our SEPMAG® Systems have homogenous Biomagnetic Separation conditions. Moreover, the magnetic force values are adapted to optimal performance for most commercial magnetic beads. As we use homogenous conditions, process validation and scale-up are straightforward.
When needed, special force values (gentler or stronger force), can be provided under request. We may help you to experimentally determine the ‘optimal’ force value for your magnetic beads suspension.
Our QCR hardware and software allows monitoring of each single biomagnetic separation step, and using the data to ensure the quality of the process.
All these benefits, in an extremely safe environment, with no maintenance nor running cost.
Related articles:
- Mistake #2 in CLIA IVD-kit manufacturing: Using bigger magnets toavoid losses
- Mistake #3 in CLIA IVD-kit manufacturing: Defining the process based purely on the separation time
- Mistake #4 in CLIA IVD-kit manufacturing: Neglecting process scalability