Once you have defined the required magnetic force, with a constant magnetic force separation device, it is simple to scale up production. Having validated the magnetic force at a small scale, the same force value can be used for a larger system, even in a different magnetic separation system. Because the conditions remain the same, efficiency (no losses) and batch consistency (no irreversible aggregation) are guaranteed.

Figure 10.1 Sepmag Q1 L equipment and monitoring software.
In constant magnetic force systems, the validated magnetic force value is translated into a constant separation speed. This is due to competition between the magnetic force and the drag force generated by the viscosity of the buffer. The separation time required will therefore be proportional to the vessel diameter when the magnetic force is radial (Fig. 10.3).
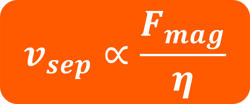
Figure 10.2 Where νsep is the separation time, Fmag is the magnetic force, and η is the viscosity.
Development and validation can be monitored by measuring the changes in absorbance (opacity) during separation. This provides users with information on the batch-to-batch consistency of the process, the performance of the re-suspension protocols, and the effects of changes on the buffer. This also gives an objective way to determine the separation time for a given vessel and magnetic force value.

Figure 10.3 Equation for scaling up the volume while keeping the magnetic force constant, where Rvessel is the radius of the vessel.
When scaling up, (e.g., from milliliters to 1 or 2 L) it is relatively simple to keep the magnetic force constant. The working volume scales up as approximately (Rvessel)3. Note that this scales exactly if the height:diameter ratio is kept constant, but the separation time would be proportional to the Rvessel. From a magnetic separation point of view, the productivity (volume of suspension processed during a given unit of time) will increase as (Rvessel)2.
Even without the need to scale up for production purposes, there are benefits to scaling up the batch volume. For example, simplifying batch validation. Using a constant magnetic force, the within-batch consistency is guaranteed. This means it is simpler and safer to validate a single large batch, than to process several batches and validate each one of them to ensure consistency between batches.
The advantages of working at a larger scale creates a need for larger biomagnetic separation systems. However, keeping the magnetic force constant for larger vessels presents some technological challenges. To keep the same magnetic force at larger volumes the permanent magnet’s weight needs to increase exponentially. For vessels larger than 5 L, this means using the same magnetic force as used for smaller vessels becomes impractical, both in weight and cost.
Alternatively, users can opt to slightly decrease the magnetic force for very large volumes. At reasonable (i.e., practical) permanent magnet weights, the lower magnetic force results in a slower separation speed. The separation time is then not only proportional to the vessel diameter, but also inversely proportional to the magnetic force. In absolute terms, the separation time is fast enough to justify the scale-up in terms of productivity.
Using classical separators, scaling up increases the likelihood of irreversible aggregation (the key limitation) especially when coating magnetic beads for IVD, as larger magnets require a larger retention force. But, using the constant magnetic force approach for scaling up, the decrease in magnetic force has the added benefit of reducing the likelihood of aggregation.

Figure 10.4 Estimate value of the separation time tsepB on a vessel of radius RvesselB in a Biomagnetic Separation Systems with constant magnetic force FmagB, calculated from the separation value tsepA values of a vessel with radius RvesselA in a system with constant magnetic force FmagA.
Estimating the separation time in two different separators with different constant magnetic forces is simple too. In contrast with the separation time experimentally determined in the first device, the separation time in the second device will be proportional to the ratio of the vessel diameters and inversely proportional to the magnetic force values (see Fig. 10.4). If the magnetic force is the same in the second system (as in certain volume ranges), then the separation time will be proportional to the vessel diameter. If the second (usually larger) system has a lower force than the first, users should correct for separation force.
Using constant magnetic force biomagnetic separation systems, you guarantee within-batch consistency. This also facilitates the smooth transfer of procedures to a different volume, which makes scaling up the manufacturing process quick and simple when production needs to increase.
The old saying ‘magnetic beads don’t work at large volumes’ is, simply, wrong. If you want to learn what leading IVD-manufacturers already know, this is the e-book for you!
Related news
- Different types of Immunoassays
- DBCO click chemistry: what is it and what are its benefits?
- What is poly dt?



