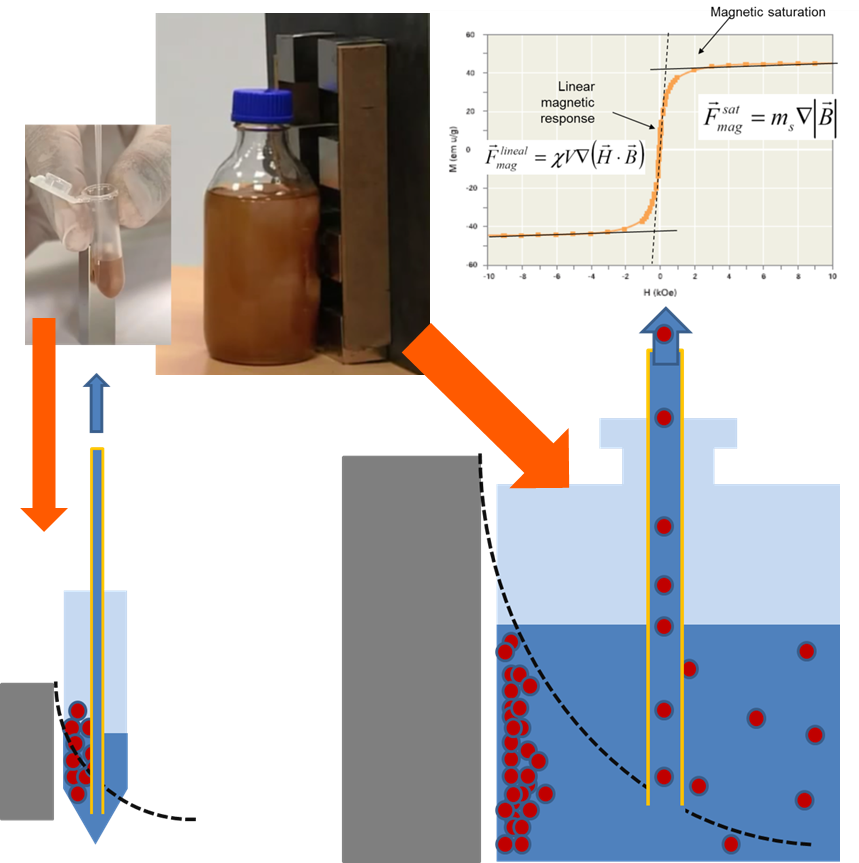
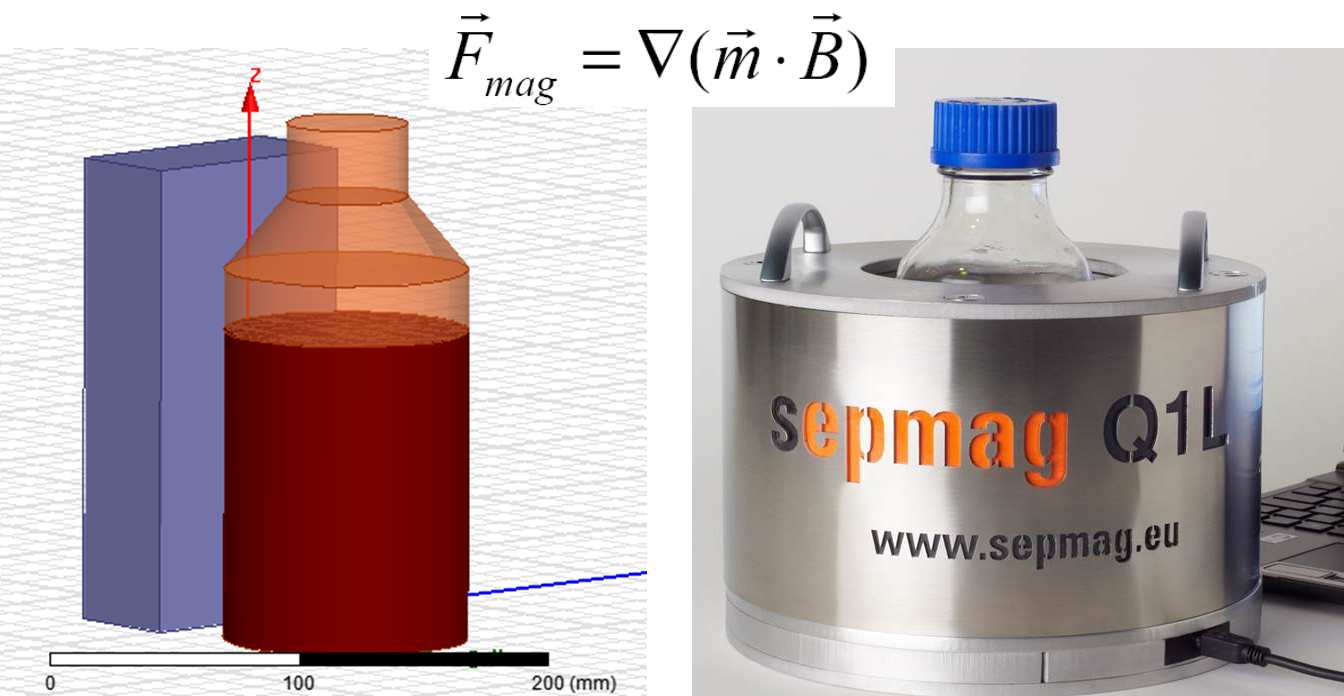
Biomagnetic separation has a growing range of applications in life sciences, from cell sorting to protein purification. For any application, correctly specified parameters are key to cost-effective, reproducible magnetic separation protocols that can be successfully scaled up from R&D to production.

Standardization, Scalability, and Quality Assurance
Establishing standard operating procedures (SOPs) for magnetic bead separation in large volumes is imperative for efficiently producing magnetic bead-based products at any scale. Traditional magnetic separators commonly used in research and development (R&D) laboratories lack standardized separation conditions due to the inherent variability in magnetic force with distance, rendering the replication of processes at larger volumes practically impossible. The dynamic nature of magnetic force, which is inversely proportional to distance squared, means that separation conditions change as soon as the vessel diameter is altered. The properties of magnetic forces make it challenging to maintain consistency in separation processes. Moreover, the option of increasing production by replicating small-volume processes is not feasible due to the high costs associated with validating multiple individual batches and ensuring their compliance with specifications.

Introduction
The evolution of magnetic bead technology has opened up a plethora of possibilities in the field of life sciences, spanning various applications from nucleic acid purification to protein isolation. However, the transition from research and development (R&D) to large-scale production has often been hindered by the lack of standardized tools used to separate specific proteins or molecules from a complex biomaterial. This bottleneck has posed challenges, particularly in industries such as in vitro diagnostic (IVD) reagents manufacturing and magnetic bead production, where scaling up protocols from R&D to production has been a daunting task. In this article, we explore how the standardization of magnetic bead separation conditions at the R&D level can help establish protocols built for a production level. Such standardization will aid in revolutionizing magnetic separation, ultimately simplifying and accelerating large-scale manufacturing.

The efficient and precise nature of magnetic beads separation has improved our ability to capture and isolate biomolecules. Biomagnetic separation protocols are straightforward too, rendering the fiddly centrifugation and filtration steps required in many processes unnecessary.

The demand for magnetic bead technology for capturing biomolecules has grown rapidly across the life sciences. This includes the use of magnetic beads in chemiluminescent immunoassays (CLIA) and molecular diagnostic tests, which has facilitated rapid improvements in sensitivity and automation in these fields. This in turn has made these tests increasingly useful and viable in clinical and diagnostic settings.
Nucleic acid (DNA and RNA) purification and amplification is an important tool for molecular biology and an important step before many biochemical and diagnostic processes. These techniques have made great progress recently[1] [2] due to the increasing number of sudden and public health-threatening infectious diseases (e.g. Ebola virus, Zika virus and more recently SARS-Covid). For quickly and reliably diagnosing these diseases, nucleic acid detection are key tools used in on-site immunological technologies and rapid test kits (for magnetic mRNA purification refer to “Oligo dT-coated magnetic beads: the benefits of their application for mRNA purification”).
Magnetic bead separation enables complexes of magnetic beads and their bound materials to be separated from a complex mixture in solution with a single magnetic separation rack. The result is an isolated solution of your target biological elements which can be enriched and concentrated through this process.

Good manufacturing practice, or GMP, is a set of standards that ensures that produced products meet a set of quality standards. Following GMP is crucial for the production of laboratory equipment, as it ensures that a manufactured product is able to meet predefined criteria. Most GMP practices follow the guidelines set by the FDA in the United States, and are promoted by the WHO.
Overview of cell biology and its importance
The main two categories of cells are prokaryotic and eukaryotic cells. Prokaryotic cells are what make up bacteria and archaea while eukaryotic cells make up organisms of the domain eukaryota. The inside of the prokaryotic cell houses it’s genetic material, DNA, in a region called the nucleoid region. There are also ribosomes within the central region of the bacteria. The next layer is the plasma membrane, a bi-lipid layer like you will see for eukaryotic cells. Unlike eukaryotic cells, prokaryotic cells will have a cell wall and a capsule surrounding the cell membrane. The eukaryotic cell is surrounded by a lipid membrane, and has membrane-bound organelles. The genetic material, DNA, is stored in the nucleus which is a membrane bound organelle. In research, many different types of cells are used. Bacterial cells are used in protein purification to grow a plasmid to express a protein of interest. You can read about this in our article protein expression and purification. Many types of Eukaryotic cells are used to do in vivo studies. Depending on your research interests, you might use muscle cells, or skin cells, or cancer cells.
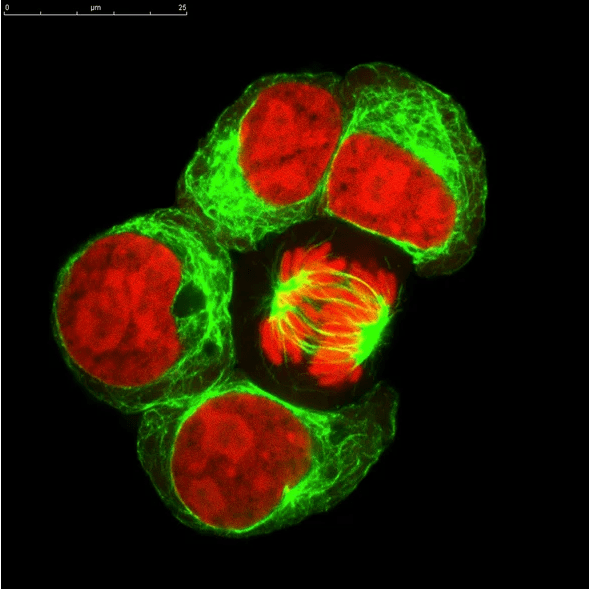
Cell based assays are used to quantify cellular function, measure how stimuli affect cells, or to localize an effect within the cell. The cells are live and intact, and require the use of fluorescent tags and chemiluminescent or colorimetric enzymes. The quantification is performed by flow cytometry or microscopy. This is very different from studies of protein or nucleic acid which require destruction of the cell and isolation of those components from cell lysate. A cell based assay is conducted entirely within live, intact cells. The goal is to understand a cellular process, localization of a molecule or drug to a cellular compartment, or to measure how cells react to a substance. Cell based assays are usually performed in tightly controlled cell lines to test for a wide range of behaviors:
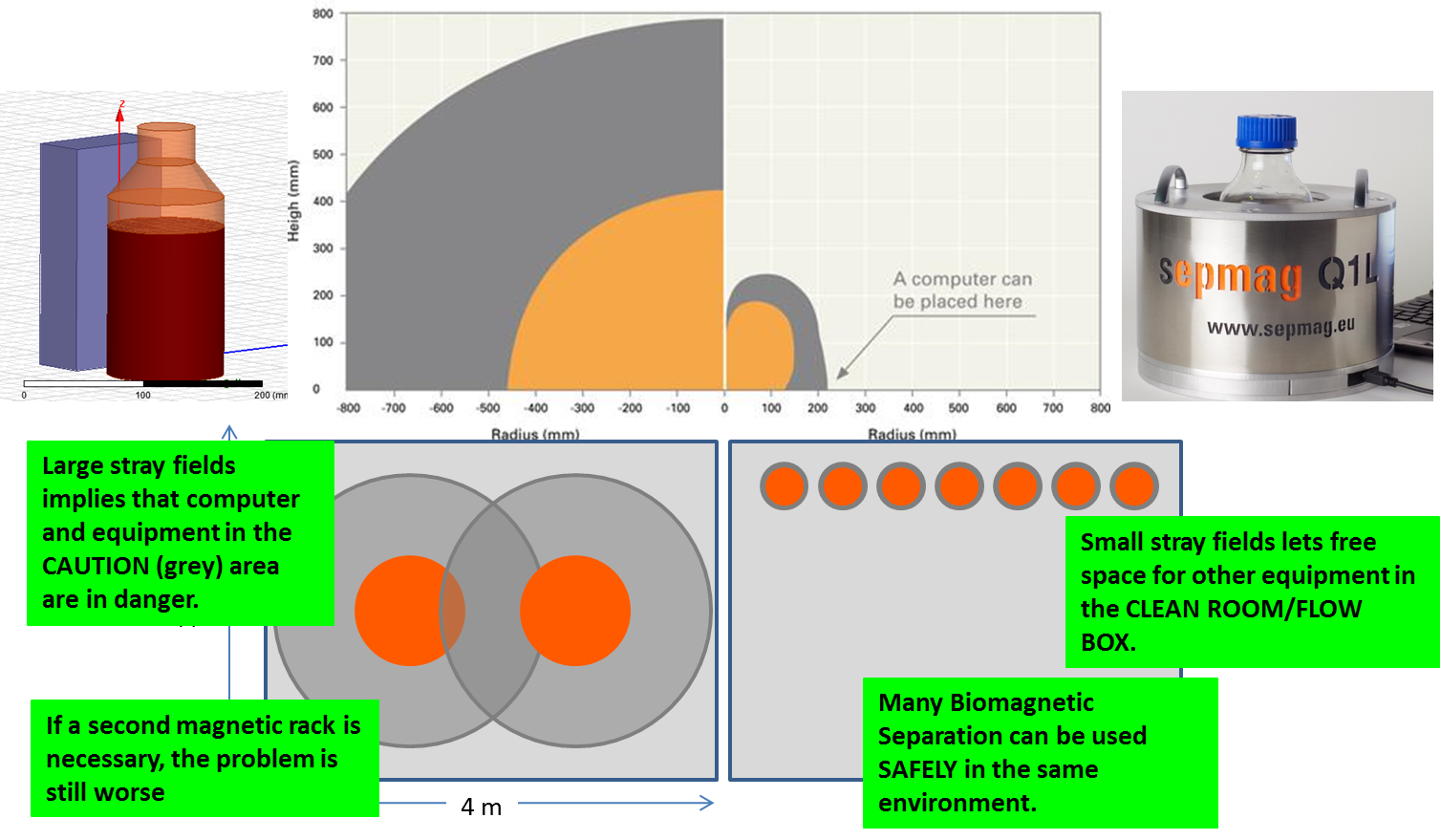
The first four mistakes we described in the last weeks are related to the production process of CLIA IVD-kits. However, even if you get a perfect reproducible, high performant process, it is a last mistake you should avoid. We have frequently see IVD-manufacturers to adopt solutions implying high safety risk for the operators and the equipment.

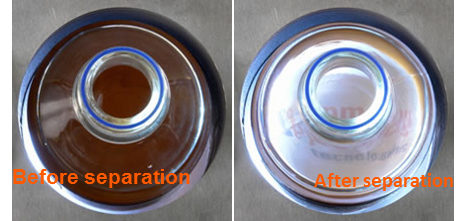
Not all mistakes made in CLIA-IVD kit manufacturing involve the magnetic rack itself. Besides the two mistakes we reviewed during the last weeks, the third mistake we have detected involves process validation. Biomagnetic separation processes are often validated solely by specifying a separation time.
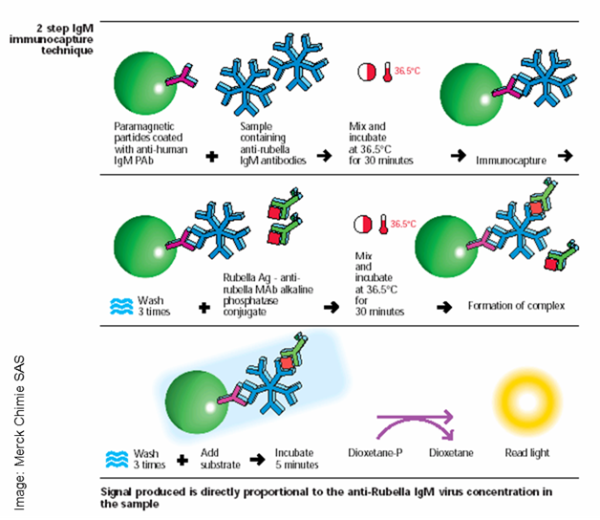
Product development is a time-consuming, expensive process for CLIA-IVD kit manufacturers. There are several steps involved:
- Selecting the biomarker
- Choosing the right coupling
- Selecting the right magnetic bead
You are well versed with the first two points but what is “the right bead”? Assuming you have the right biomarker and a perfect coupling, the ideal magnetic bead should have the following properties:
- High recovery/fast separation, compatible with the timing of the analyzer step. It needs to be fast enough during large-scale production processes without high bead and coupled biomarker losses.
- No aggregation problems. Beads should be easy to re-suspend. It makes no sense to separate quickly if several additional sonication steps are required, which are difficult processes to control/implement in large volumes.
- Low kit-to-kit variability. Batch aliquots (typically less than a milliliter) of production batches (liters scale) must be consistent. If not, variability causes problems when interpreting the results in the analyzer.
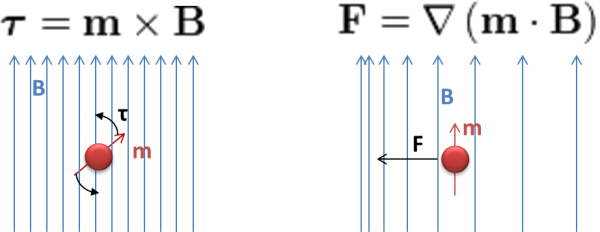
When a new CLIA-IVD kit is transferred from R&D to production, all the manufacturing protocols should be adapted to the new throughput and volume. Biomarker specifications, buffers and coating protocols would benefit from the cumulated experience in non-magnetic kits. Coupling an antibody to magnetic beads is quite similar to doing it in colloidal gold or latex particles. But the washing protocols using Biomagnetic Separation are something new. The use of classical (and dirty) centrifugation method makes not so much sense when we can use the magnetic properties of the beads. Similar reasoning applies to the use lateral flow filtration or other complex and time-consuming non-magnetic separation techniques.

The most frequent concern when considering the use of modern Biomagnetic Separation Systems is their compatibility with specific magnetic beads. Although Sepmag® devices are already working successfully in IVD labs and production lines, this is a very legitimate question.
Learning more about the behavior of magnetic beads separation when strong magnetic fields are applied

When one scales up production using a classic magnetic separation system, one finds that the separation time increases quickly with an increase in production volume. An increase in separation time means that material losses are higher and aggregation problems become a serious problem. By using homogenous separation time, one finds that the magnetic separation process is shorter and the separation time can be easily estimated. In homogeneous systems material loss and bead aggregation is minimized.


Magnetic beads need to be constantly mixed and homogenized to avoid sedimentation and clumping problems. Even when the sonication method is necessary to break up aggregates, mixing and homogenization is necessary.
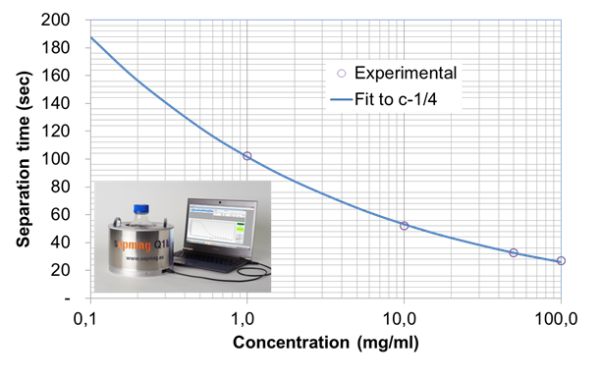
In the Life Sciences, one of the most critical parameters for final IVD kit performance is magnetic bead concentration. The beads are functionalized before the magnetic separation process with antibodies or other biological molecules, so the concentration of magnetic beads also delivers a specific concentration of biologically active reagent. If you do not have the correct amount of beads/biological molecules in your preparation, the sensitivity of the kit changes significantly. Therefore volume control of the suspension is quite important.


In non-homogenous magnetic separators, monitoring the entire separation process is difficult to impossible. As a result, errors in the magnetic separation process, such as using the wrong magnetic beads or using buffers with the wrong properties are not detected until the final QC testing stage.

When magnetic bead reagents are produced in quantity, often you cannot know if you have the correct properties of the beads until the final quality control step. But if these properties are wrong, finding out the properties at the end of the magnetic separation process for production does not allow you to salvage the lot. Knowing magnetic bead properties, such as size, magnetic charge and surface charge, is critical in order to have excellent reproducibility in the final product (e.g. IVD kits).

Classic magnetic separation equipment requires a large amount of space in order to comply with health and safety regulations. While the magnetic separation process has numerous advantages, the magnetic fields surrounding the devices may be so large that they fall within the ‘danger’ and/or ‘caution’ areas.

The separation time in standard magnetic separation devices is usually determined by analyzing aliquots of solution taken at different times. The problem is that each aliquot gives the technician information about one spatial point in time. Therefore, the design of validation experiments becomes a very complex endeavor.


When scaling up a process using a traditional magnetic separation rack, the percentage of bead and biomolecule losses significantly increases with an increase in volume. One way of dealing with this problem is by applying a higher force at longer distances. But for this to work, you must apply this greater force without increasing the forces in the retention area during the magnetic separation process, in order to avoid irreversible aggregation.


If one wants to scale up production from small lab lots to full-scale large lots, a non-homogenous magnetic separation process will result in lot-to-lot inconsistencies. Homogenous biomagnetic separation conditions, however, guarantee consistent results regardless of production scale.

Biomagnetic separation techniques are faster, cheaper and easier to use than non-magnetic techniques. In addition, when a magnetic separation process is performed under homogenous conditions, these techniques are also scalable and easily validated.
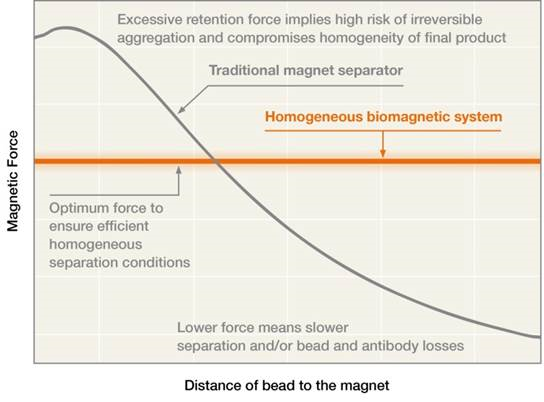
Due to the inherent properties of classic non-homogenous biomagnetic separators, beads can aggregate during the magnetic separation process. When this happens, technicians try to resolve the magnetic beads separation problem by using special resuspension techniques like the sonication method. But problems with resuspension can ultimately lead to end-product variability, especially if aggregation is not detected early.
By Josep Maria Simó, CEO Sepmag

When using biomagnetic separation, in order to ensure the consistency of the resulting product and the process itself, there must be some sort of validation procedure. Validation should be consistent within a given lot, from lot to lot and also when the process is scaled up. The validation procedure should optimally be related to the conditions of magnetic bead separation and not be dependent on any specific device that generates the magnetic field.
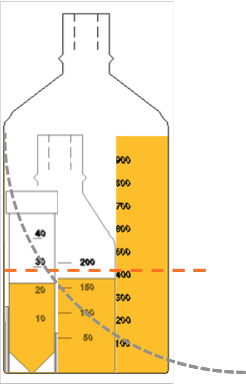
Biomagnetic separation used to take place in academic labs, but recently it has become a very industrial application. As processes are scaled up and volumes increase, the investment required for each batch is larger, but the expected economic return is also larger.

Because biomagnetic separation techniques are relatively simple, life science laboratories and industries are quite enamored with them. Indeed, using only magnetic beads and magnetic fields, biomolecules can be captured and extracted from complex media in magnetic bead separation. However, if this application is to be considered practical, it should also be faster than other separation technologies such as chromatography, electrophoresis or centrifugation.

Because biomaterial is expensive, fragile, complex and sometimes rare, biotech companies spend a great deal of time and resources to develop and refine biomaterial production processes. Quality control and standard operating procedure demand that production managers make sure that all technicians and operators know and follow the exact procedures from batch to batch.

Consistent lot-to-lot results are achieved with biomagnetic technology only when magnetic bead separation is performed in defined and homogeneous conditions. When homogeneity is realized, separation is reproducible and scalable.

Of course, as in most industries, product consistency is key to the success of the Life Sciences industry. With magnetic bead separation, not only should working conditions be constant over time, but conditions should also be consistent from lot to lot, regardless of the time between production runs. One thing that should always be considered is the quality of the magnet used in the biomagnetic separation devices.
Those who use Life Sciences products rightly demand that these products show consistency from batch to batch. In other words, when comparing batches, one should find very little, if any, variability.
One of the major problems of traditional magnetic bead separation technology is aggregation of the magnetic beads. Aggregation decreases the yield and also contributes to variability between batches.

Reproducibility is very important when considering production of in vitro diagnostic kits. As such, there should be good quality control in place that strictly defines the parameters of the assay’s raw materials. For example, magnetic beads, antibodies and buffers should not vary from batch to batch. In addition, these raw materials should all act the same during biomagnetic separation.
Often in life science research and clinical applications, magnetic carriers are utilized to separate or isolate biomolecules from suspension. A biomolecule is coated onto a magnetic bead or is ‘captured’ by a bead and then pulled to a given position in the solution via magnetic forces. In order to establish a robust and reproducible standard operating procedure (SOP), one needs to understand how the magnetic forces are generated and what key parameters need to be controlled.

When a biotech company or other related industry decides to use biomagnetic separation technology, a great deal of time, energy and resources are typically spent by R&D and manufacturing departments to determine the optimal bead to use for their specific applications. Often, however, they overlook one of the major determinants of separation: homogeneity of separation conditions.

Magnetic beads are used by many biotech companies, typically for in vitro diagnostic applications and magnetic bead separation. Because these beads are so widely used, it is very important that every aliquot of every batch has exactly the same properties.

Biomagnetic separation needs validation in order to ensure reproducibility. The skills necessary to identify the key parameters affecting separation performance, measure those parameters, and enact the appropriate controls are specific and require an excellent background in physics.

Magnetic bead separation is being used in more and more applications (e.g. immunoassays, collection of genetic material, protein purification). Most of these applications are industrialized and therefore require quality control protocols, validation audits and standard operating procedures. Because of the economic implications, production mistakes are not acceptable, so products must demonstrate reproducibility in order to be viable.

The cornerstone of any good production process is the ability to have robustness and reproducibility, especially in the biotech industry and in the magnetic bead separation industry.

Since reproducibility over time is a highly desired trait when using biomagnetic separation, especially when used in the life sciences, it is important to consider all possible disruptions of consistency. Biomagnetic separation devices use permanent magnets which maintain their properties over long periods of time.

Biotech companies such as InVitro Diagnostic aim for 100% reproducibility in every single batch they produce and in every kit test in each batch. In fact, customers expect that they will receive a product that will perform exactly the same as the last time they purchased it. It does not matter what batch the product is or when it was produced.

It is vitally important for life sciences products to be consistent from lot to lot, so two batches of the same product produced in the same way should have little variability. In order to achieve a high level of quality control, one must define a strict standard operating procedure (SOP). In the case of a permanent magnet magnetic bead separation device, conditions are usually very stable and so the main parameter to control is the time the vessel is exposed to the magnetic field during production.

There are no easy ways to bypass steps or simplify the production process of magnetic bead separation. Steps in the production process that seem easy or easily bypassed, turn the production into a nightmare if one attempts to take short cuts. One of these seemingly easy steps is the resuspension step.
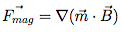
During the development of a magnetic bead separation process, scientists put great effort into reproducing the size of the beads, the magnetic charge on the beads, buffer composition, pH and temperature. What is often overlooked, however, is the importance of homogeneous biomagnetic separation conditions.
This post is about magnetic bead separation and how to validate this process. If you are interested in this topic, and are willing to learn more about it, download our Free Guide The Starting Guide to Validate Biomagnetic Separation Processes:

Separation techniques using magnetic carriers (either beads or particles) are often used in the life sciences to ‘capture’ specific biomolecules. These techniques utilize immunocapture, DNA fragments, or electrical charge in order to specifically target the biomolecule of choice. After magnetic capture of the biomolecule, magnetic forces can separate it from the rest of the milieu. Because of the seeming ease of separation, biomagnetic techniques are used by some as the ‘gold standard’ of separation technology.

Biomagnetic separation technology is being widely adopted in many biotech and other life science industries. The managers of these industries spend a lot of energy, time and resources choosing the correct beads for their applications, however they often overlook very important variables that need to be controlled pertaining to the magnetic bead separation process.

In Vitro Diagnostic (IVD) Immunoassays are some of the most successful life science magnetic bead and particle research applications that have come to market. The demand for such technology in hospitals and laboratories in particular, has grown dramatically.

It is common knowledge that industries and biotech companies must validate any production process in order to maintain the consistency of their products. In processes that cannot be validated at a high level, lot numbers become very important so that the end user can compare and understand lot to lot variation.
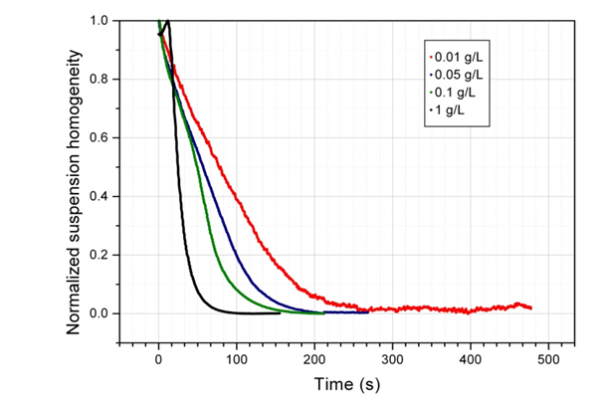
Non-homogeneous biomagnetic separation processes are difficult to validate and standardize. The fact that magnetic beads move at different speeds due to the varied magnetic forces in a non-homogeneous environment makes monitoring the production almost impossible.