Magnetic properties of nanoparticles are used for drug delivery, therapeutic treatment, contrast agents for MRI imaging, bioseparation, and in-vitro diagnostics. These nanometer-sized particles are superparamagnetic, a property resulting from their tiny size—only a few nanometers—a fraction of the width of a human hair (nanoparticles are approximately 1/1,000 thinner than human hair). Superparamagnetic nanoparticles are not magnetic when located in a zero magnetic field, but they quickly become magnetized when an external magnetic field is applied. When returned to a zero magnetic field they quickly revert to a non-magnetized state. Superparamagnatism is one of the most important properties of nanoparticles used for biomagnetic separation.
How nanoparticles are made
Magnetic nanoparticles are usually based on magnetite and/or maghemite, two different forms of iron oxide. The most popular way of obtained it is co-precipitation, where the material is directly synthesized as spherical particles. Controlling the synthesis parameters, it is possible to achieve sizes of less of 10 nm. Researchers and industrials are continuously improving the chemical or physical methods to achieve a better control of both composition (ratio magnetite/maghemite) and size and thus, the magnetic properties.
Applications of nanoparticles
Individually or as composites, the unique features of nanoparticles allow them to be used for a variety of applications:
- Carriers: Nanoparticles can be used to deliver molecules to targeted locations. This can be for the purpose of diagnosis or for drug delivery.
- Biomedical devices: Innovators in the biomedical field are using nanoparticles for diagnostic array development. When combined with fluorescent tagging technology the particles are also used for imaging.
- Food safety: Nanoparticles have been used for creating packaging for better food preservation and for more targeted use of pesticides in food growth.
- Biomedical research: nanoparticles of the magnetic variety are commonly used laboratories for magnetic separation of molecules.
Advantages of nanoparticles
Nanoparticles hold many advantages in their various uses. The particles have a large surface to volume ratio, making them efficient for use across disciplines. They can be made soluble in water, and not toxic to the body for effective use as a carrier in the human body. With externally applied stimuli, magnetic nanoparticles hold great power for targeting, imaging and separation of molecules. Magnetic nanoparticles can host a multitude of surface conditions while reacting to outside stimuli very precisely.
Magnetization of superparamagnetic properties of nanoparticles
Magnetization is defined as the extent to which a material becomes magnetized when placed within a magnetic field, and is a measure of the net magnetic dipole moment per unit volume. The magnetization is directly related to the applied magnetic field.
Magnetic susceptibility (χ) is a dimensionless quantity that indicates the degree of magnetization of a material in response to a magnetic field. Magnetic susceptibility is equal to the ratio of magnetic dipole moment to magnetic field.
- M=magnetization (magnetic dipole moment per unit volume)
- B=applied magnetic field
- χ=magnetic susceptibility
χ =M/B
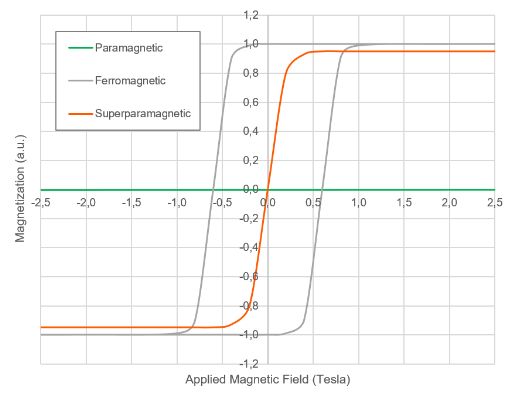
Non-magnetic materials, like water, are diamagnetic and experience a small negative response to the magnetic field. In the case of diamagnetic materials, the susceptibility is negative and has a value around -10e-5. Paramagnetic materials, like oxygen molecules, experience a slightly positive response to the magnetic field. In the case of paramagnetic materials, the magnetic susceptibility is around 10e-4 or 10e-5. In both cases, the magnetic dipoles attempt to align with the external magnetic field, and fight against thermal agitation. Diamagnetic and paramagnetic dipoles don’t interact with each other, and their susceptibility at room temperature is very small.
The situation is very different for magnetic materials, which are technically defined as ferro and ferrimagnetic materials. In magnetic materials, each dipole interacts with its nearest neighbors. This inter-dipole interaction creates a high magnetic response throughout the material, with susceptibilities that can reach values higher than 10e3. The drawback is that the materials show magnetic hysteresis: after applying a magnetic field, the material will have ‘memory’, meaning that when the applied field returns to zero the material remains at a certain magnetization.
This is a very useful property for developing permanent magnets, but it is a problem for many Life Science applications. Ferromagnetic beads will remain magnetized after the removal of an applied magnetic field, and the beads will form irreversible clumps or aggregates. The ideal material for biological applications would have a high magnetic susceptibility, but no magnetic ‘memory’. They would combine a high response to applied magnetic field with a non-magnetic behavior once the magnetic field is removed.
Advances in nanotechnology have provided a way to obtain these superparamagnetic properties of nanoparticles by reducing the size of the ferro- or ferrimagnetic material to few nanometers (below the so-called superparamagnetic diameter). When they are below the superparamagnetic diameter the nanoparticles are able to return quickly to a non-magnetized state after an external magnet is removed. Larger ferro- and ferrimagnetic materials have remnant magnetism after the applied magnetic field returns to zero.
Magnetic nanoparticles for use in research and industry
Now that you have learned all about the magnetic nanoparticle technology, let us discuss in more detail how they are used. For example, magnetic nanoparticles can be used to purify antibodies from a mixture in solution. These magnetic nanoparticles are pre-conjugated to a molecule that specifically binds antibodies, or immunoglobulins. These types of molecules are called either “protein A” or “protein G”, as well as a hybrid called “protein A/G”. You choose which one you use based on the subclass of antibody you are interested in purifying and from which organism it comes from.
Once the antibodies bind to the magnetic particles, also called magnetic beads, you can change out the liquid in the container by placing it in a magnetic rack. A magnetic rack will keep the magnetic particle bound to your antibodies in place, while the liquid can be removed. You can wash the particles while they are bound by the magnetic rack. Lastly you can elute the antibodies from the beads.
One of the limitations of this technique in the past was the size of the magnetic rack that is available. Magnetic racks are now available in a large variety of sizes from milliliter to tens of liters in size. This makes the technique useful from a laboratory scale to the scale of industry.
Related news