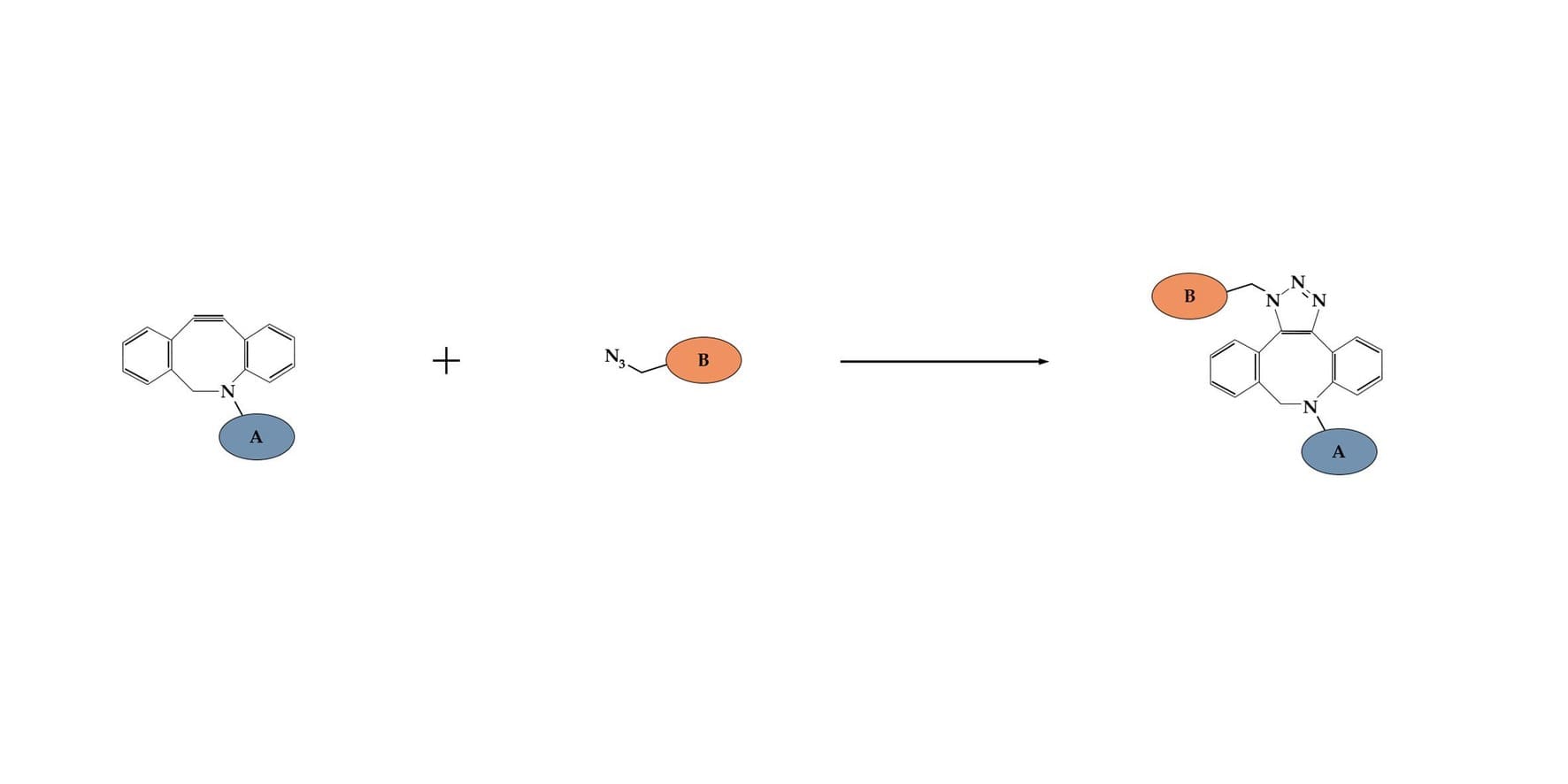
DBCO click chemistry is a type of reaction that has underpinned recent advances in biomedical research, as well as pharmaceuticals and biotech. Copper(I)-free click chemistry, such as DBCO click reactions, enable fast and specific conjugation of target molecules under aqueous conditions and do not require toxic catalysts.
What is meant by click chemistry?
Click reactions are a two-step technique where two molecules are conjugated, “click”, by selectively reacting with each other. Click chemistry reactions fall into two main groups: Copper(I)-catalyzed Azide-Alkyne (CuAAC) reactions, or copper-free strain-promoted azide–alkyne cycloaddition (SPACC) reactions. Strain-promoted reactions that use cyclootynes produce stable products, high yields and have efficient reaction processes.
What is DBCO click chemistry?
There are many different types of click chemistry reactions, which use a wide range of reagents. Dibenzocyclooctyne (DBCO) click chemistry uses a DBCO compound, a strained cyclooctyne derivative, to join target molecules. DBCO reagents, also known as ADIBO and DIBAC, are the most commonly used substrates for copper-free click chemistry.
How does DBCO click chemistry work?
DBCO click chemistry compounds react with an azide-functionalized compound or biomolecule, conjugating them to form a stable triazole link. A copper(I) catalyst isn’t required, due to the lower activation energy of DBCO. This method can be used to rapidly and selectively join ligands, such as antibodies, peptides or proteins, to the surface of liposomes. The reaction, called a cycloaddition reaction, is made up of three key steps:
- Activation (functionalization) of biomolecule A, such as an antibody, with DBCO.
- Activation of biomolecule B, such as a liposome, with an azide group.
- Joining of molecules A and B to form a conjugate, e.g. antibody conjugated liposomes. These two molecules are joined via a triazole linker.
Advantages of DBCO click chemistry
While early click reactions were copper-catalyzed reactions, the cytotoxic nature of the copper catalyst limited the applications of click reactions. The copper-free, efficient, and specific nature of DBCO click chemistry reactions has several advantages:
Specific reactivity
DBCOs have a narrow and highly specific reactivity towards azides, meaning they only react with azides.
Bio-orthogonal: suitable for in vivo uses
DBCO click chemistry reactions are bio-orthogonal, meaning they are inert to groups such as amines and hydroxyls, which naturally occur in many biomolecules. As a result, DBCO click reactions can occur within organisms without interfering with biochemical processes. This allows specific cellular target proteins to be labeled, and drug-target interactions to be studied in live cells. DBCO click reactions can also be used to develop molecular tools to investigate tissue development, and diagnose and monitor disease.
Biocompatible: non-toxic
Using DBCOs lowers the activation energy of the cycloaddition click reaction, allowing it to be carried out without a cytotoxic copper catalyst.
Stable product
The triazole linker that forms during a cycloaddition reaction is very stable. As a result, DBCO click chemistry is more efficient than copper-catalyzed reactions.
No complex assay optimization
The absence of a copper catalyst means that time-consuming optimization of assay conditions is not needed for DBCO click chemistry.
Rapid and quantitative labeling
The DBCO click chemistry reaction results in a high yield of stable triazoles in a short amount of time. The efficiency of the labeling means that the products are also highly homogeneous.
Applications of DBCO click chemistry reactions
DBCO-containing reagents come in a wide range of forms, suitable for different applications, including:
Fluorescent labeling
DBCO-containing fluorescent dyes can be used for fluorescent labeling of azide-tagged molecules. DBCO click chemistry can also be used to label molecules such as proteins with fluorescent tags, and then graft these to magnetic beads and capture them with a magnet.
Immobilizing enzymes
Click chemistry can be used for creating immobilized proteins, such as enzymes, where covalent bonds between amino acid groups and the enzyme are used to control the enzyme’s orientation.
Purifying and studying DNA and RNA
DBCO magnetic beads can covalently capture azide-tagged biomolecules on a magnetic bead matrix. The beads and attached proteins can then be washed, eliminating non-bound proteins and purifying the target protein. This method can purify DNA, RNA, proteins, cells and plasmids. Recent research has demonstrated that DBCO magnetic beads can be used to study DNA-protein complexes that are extracted from cell lysate – something that is otherwise difficult to do.
Future applications of DBCO click chemistry
Labeling biomolecules requires protocols that can be carried out under biocompatible conditions: neutral pH, aqueous solution and ambient temperature. DBCO click chemistry meets these criteria, enabling non-toxic, efficient, selective and low background detection and labeling of azides.
Exciting applications of DBCO click reactions are still being uncovered, from in vivo cartilage regeneration to developing biomaterials, and its ability to be used alongside magnetic separation systems widens the scope of this potential.