The demand for magnetic bead technology for capturing biomolecules has grown rapidly across the life sciences. This includes the use of magnetic beads in chemiluminescent immunoassays (CLIA) and molecular diagnostic tests, which has facilitated rapid improvements in sensitivity and automation in these fields. This in turn has made these tests increasingly useful and viable in clinical and diagnostic settings.
With the large number of polymerase chain reaction (PCR) tests carried out during the COVID-19 pandemic, magnetic beads have also been increasingly widely used as solid support for immunoaffinity purification. For example, magnetic beads have served as a solid support during RNA-based vaccine manufacturing, delivering faster and more efficient processes. Similarly, many biotech companies are now exploring magnetic purification as an alternative for improving their downstream processes, especially for proteins isolated from impure raw suspensions.
Much of this success is due to the ability of magnetic beads to be mobile during the capture of biomolecules, yet almost instantly become immobilized upon applying a magnetic force. This level of control is not possible with other widely used methods and technologies, which makes working with biological suspensions through magnetic bead technology easier and more precise.
However, the expansion in new applications for magnetic bead technology has brought many scientists into contact with magnetic separation protocols for the first time, and there are some common issues that new users often experience. In addition, after successfully utilizing magnetic bead-based biomolecule isolation at the research and development (R&D) scale, many new users look to translating this into larger scale manufacturing processes. The differences between magnetic bead technology and other technologies means that scaling up these protocols can be challenging for new users.
Within the fields of reagents manufacturing and product purification, for example, technical staff are responsible for standardization, safety, quality control and the scale up of protocols. Scientists should be highly skilled at developing robust protocols, taking into consideration the surface chemistry of the beads, the batch volumes and concentration, the composition of buffers, and the incubation time and temperature.
However, for scientists unfamiliar with key magnetic properties, it is easy to overlook parameters that need to be defined for biomagnetic separation protocols, and this can undermine the success of the process. In particular, it can make it extremely difficult to reproduce, or scale up from, initial small-scale protocols. This means R&D departments often face a bottleneck when transferring protocols to the production phase.
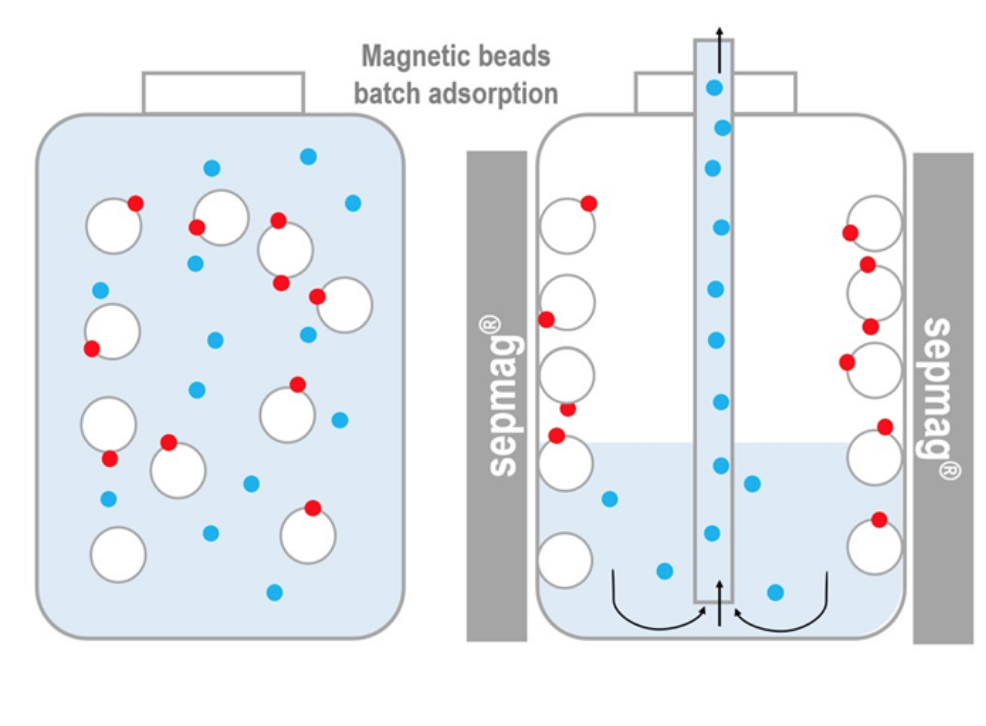
Magnetic beads separation process. The magnetic beads coated with a ligand capture the target molecule. Once the suspension is introduced in a magnetic separator, the magnetic beads (with the captured target molecule) are immobilized in seconds and the supernatant can be completely removed.
Properly defined magnetic separation protocols have been utilized for more than fifteen years for in vitro diagnostic (IVD) reagent manufacturing and magnetic bead production. This has allowed manufacturers to successfully scale up from milliliter to tens of liter sized batches. By incorporating a few key concepts of magnetism, it is very possible to successfully work at scale with magnetic bead technology. Armed with this knowledge, technical staff can fully characterize and validate magnetic separation processes, and by doing so standardize magnetic separation conditions and develop robust protocols that can be easily transferred to larger volumes.
In this e-book, we will discuss the parameters that are often forgotten during biomagnetic separation processes and why they matter. We will also explore the problems users have when scaling up their protocols and how to solve them. So, if you have had success at small scale batches, and want to find out how to increase your consistent biomagnetic separation protocols to any scale, this e-book is for you.

Related news:
- Targeted Magnetic Microsphere Delivery of DNA to Encourage Vascular Endothelial Growth
- Controlling Mesenchymal Stem Cell Growth with Magnets in Lieu of a Scaffold
- Magnetic Particles in Fluorescence Activated Cell Sorting
Published on March 11, 2015 and updated on February 22, 2024.





